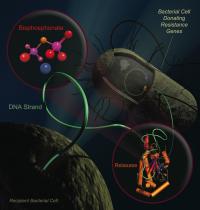
Antibiotic resistance propagates in bacteria by moving DNA strands containing the resistance genes to neighboring cells. An enzyme called relaxase is essential for this process. Bisphosphonates, already approved to treat bone loss, have now been shown to potently disrupt the relaxase function. Some bisphosphonates prevent the transfer of antibiotic resistance genes and selectively kill bacterial cells that harbor resistance. Credit: Scott Lujan, University of North Carolina at Chapel Hill Putting bacteria on birth control could stop the spread of drug-resistant microbes, and researchers at the University of North Carolina at Chapel Hill have found a way to do just that.
The team discovered a key weakness in the enzyme that helps “fertile” bacteria swap genes for drug resistance. Drugs called bisphosphonates, widely prescribed for bone loss, block this enzyme and prevent bacteria from spreading antibiotic resistance genes, the research shows. Interfering with the enzyme has the added effect of annihilating antibiotic-resistant bacteria in laboratory cultures. Animal studies of the drugs are now underway.
“Our discoveries may lead to the ability to selectively kill antibiotic-resistant bacteria in patients, and to halt the spread of resistance in clinical settings,” said Matt Redinbo, Ph.D., senior study author and professor of chemistry, biochemistry and biophysics at UNC-Chapel Hill.
The study appears online the week of July 9, 2007, in the Proceedings of the National Academy of Sciences. Funding was provided by the National Institutes of Health.
The study provides a new weapon in the battle against antibiotic-resistant bacteria, which represent a serious public health problem. In the last decade, almost every type of bacteria has become more resistant to antibiotic treatment. These bugs cause deadly infections that are difficult to treat and expensive to cure.
Every time someone takes an antibiotic, the drug kills the weakest bacteria in the bloodstream. Any bug that has a protective mutation against the antibiotic survives. These drug-resistant microbes quickly accumulate useful mutations and share them with other bacteria through conjugation – the microbe equivalent of mating.
Conjugation starts when two bacteria smoosh their membranes together. After each opens a hole in their membrane, one squirts a single strand of DNA to the other. Then the two go on their merry way, one with new genes for traits such as drug resistance. Many highly-drug resistant bacteria rely on an enzyme, called DNA relaxase, to obtain and pass on their resistance genes. A mutation that provides antibiotic resistance can sweep through a colony as quickly as the latest YouTube hit.
The researchers analyzed relaxase because it plays a crucial role in conjugation. The enzyme starts and stops the movement of DNA between bacteria. “Relaxase is the gatekeeper, and it is also the Achilles’ heel of the resistance process,” Redinbo said.
Led by graduate student Scott Lujan, the team suspected they could block relaxase by searching for vulnerability in a three-dimensional picture of the relaxase protein. Lujan, a biochemistry graduate student in the School of Medicine, confirmed the hunch using x-ray crystallography, which creates nanoscale structural images of the enzyme.
The researchers predicted that the enzyme’s weak link is the spot where it handles DNA. Relaxase must juggle two phosphate-rich DNA strands at the same time. The team suspected a chemical decoy – a phosphate ion – could plug this dual DNA binding site. Redinbo, who has a background in cancer and other disease-related research, realized that bisphosphonates were the right-size decoy.
There are several bisphosphonates on the market; two proved effective. The drugs, called clodronate and etidronate, steal the DNA binding site, preventing relaxase from handling DNA. This wreaks havoc inside E. coli bacteria that are preparing to transfer their genes, the researchers found. Exactly how bisphosphonates destroy each bacterium is still unknown, Redinbo said, but the drugs are potent, wiping out any E. coli carrying relaxase. “That it killed bacteria was a surprise,” he said. By targeting these bacteria, the drugs act like birth control and prevent antibiotic resistance from spreading.
Redinbo, who cautions that the results only apply to E. coli, said further testing will reveal whether bisphosphonates also attack similar species like Acinetobacter baumannii (hospital-acquired pneumonia), Staphylococcus aureus (staph infections) and Burkholderia (lung infections).
“We hope this discovery will help existing antibiotics or offer a new treatment for antibiotic-resistant bacteria,” he said.
The drugs may be most effective at sites where clinicians can best control dosage – on skin and in the gastrointestinal tract, Redinbo said. Other applications may include disinfectants and treatments for farm animals.
Study coauthors, all from UNC-Chapel Hill, include Laura Guogas, Heather Ragonese and Steven Matson. Redinbo is a member of the UNC Lineberger Comprehensive Cancer Center.
Redinbo and his colleagues have filed a patent and formed a small company to further develop the technology. Source : University of North Carolina at Chapel Hill
 Print Article
Print Article Mail to a Friend
Mail to a Friend
