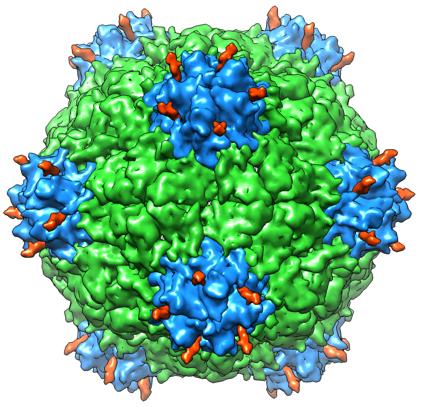
A new study shows that a hollowed-out version of cowpea mosaic virus could be useful in human therapies. Viruses aren't always bad. In fact, scientists can harness the capabilities of some viruses for good--modifying the viruses to carry drug molecules, for example.
One useful virus has been cowpea mosaic virus (CPMV), a plant pathogen that can be modified to aid in tumor detection and even chemotherapy.
In a new study, published online ahead of print in the journal Structure, researchers at The Scripps Research Institute (TSRI) report that, based on its structure, a hollowed-out version of CPMV could also be effective in human therapies.
"By studying the structure of the viral particles, we can get important information for transforming this plant virus into a useful therapeutic," said TSRI Associate Professor Vijay Reddy, senior author of the study.
An 'Empty' Virus
TSRI researchers have studied CPMV for decades. In fact, the structure of the virus was first determined in the lab of TSRI Professor Jack Johnson, who also served as a co-author on the new study.
CPMV is an especially useful drug delivery agent because it has about 300 different sites on its external and internal surfaces where researchers can attach molecules. Because CPMV is a plant virus, it is harmless to humans. To eliminate any lingering concerns of viral genomes entering the human body, scientists have created "empty" versions of CPMV, called eVLPs (empty virus-like particles), which lack the virus's genetic material.
"The eVLP is no longer a virus; it is just a protein capsule," explained Reddy.
The question has been whether eVLPs of CPMV retain the same structure in the absence of viral genome as natural CPMV viral particles.
Able to Carry the Load
In the new study, Reddy and his colleagues used an imaging technique called x-ray crystallography to create a high-resolution image of the 3D structure of CPMV eVLPs.
The image showed the structures of eVLP particles are very similar to CPMV particles, giving scientists the go-ahead to use the same modification strategies on both. This finding was in sync with a previous study showing eVLPs at a lower resolution.
The current study also revealed a new detail on both eVLPs and CPMV virus particles. Mass-spectrometry-based proteomics analysis identified multiple proteolytic cleavage sites--a spot where amino acids are cut off--on one of the proteins on the particle surface. Previous research had indicated only one such cleavage site in this region, not three. With the new information, researchers know not to add crucial molecules to those amino acids in case they get clipped off too.
Reddy said the new study opens the door to future research on using eVLPs to carry drug molecules and designing customized vaccines.
Source: Scripps Research Institute
 Print Article
Print Article Mail to a Friend
Mail to a Friend
