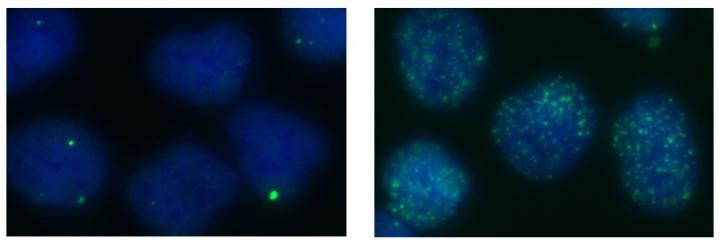
The scientists found that genome instability increases in cells as XPG levels decrease. The green spots mark locations of DNA double-strand breaks. If you have a soft spot for unsung heroes, you'll love a DNA repair protein called XPG. Scientists from the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) discovered that XPG plays a previously unknown and critical role helping to maintain genome stability in human cells. Their findings also raise the possibility that the protein helps prevent breast, ovarian, and other cancers associated with defective BRCA genes.
The research, which is published online Jan. 28, 2016 in the journal Molecular Cell, indicates XPG is essential to our health in ways far beyond it's been given credit for.
"We discovered a new function for an "old" repair protein that shows the protein is key to genome stability, and is probably important for suppressing breast and ovarian cancer," says Priscilla Cooper of Berkeley Lab's Biological Systems and Engineering Division. She conducted the research with Kelly Trego and several other Berkeley Lab researchers, and scientists from Colorado State University, Yale University, and Erasmus University Medical Center in the Netherlands.
Scientists have known for years that XPG helps carry out a DNA repair process that activates when only one of DNA's two strands is damaged. The process, called nucleotide excision repair, removes DNA lesions caused by sun exposure, chemotherapy, and other sources.
Now, to their surprise, the Berkeley Lab scientists discovered XPG is also instrumental in a process called homologous recombination, which repairs breaks on both DNA strands in cells that are copying their genomes to get ready to divide. Double-strand breaks are especially perilous to an organism because they can lead to genome rearrangements in a cell.
The scientists learned that XPG interacts with at least five other cellular proteins, including BRCA1 and BRCA2, to carry out homologous recombination. Defects in genes that express BRCA1 and BRCA2 are known causes of breast, ovarian, pancreatic, and prostate cancer. Their new study shows that cells with reduced levels of XPG have a much higher prevalence of genome instability in the form of cell cycle defects, DNA double-strand breaks, chromosomal abnormalities, and other problems.
The first hint that XPG does something in addition to nucleotide excision repair came about two decades ago when scientists learned that particular mutations in the gene that codes for XPG cause an extremely rare and devastating premature aging disorder called Cockayne syndrome. Nucleotide excision repair is not implicated in Cockayne syndrome, so scientists knew XPG must play a role in another fundamental cellular process. What that function was, however, remained a big mystery.
"We've spent the past several years searching for this unknown function of XPG," says Trego.
To begin their search, the Berkeley Lab scientists reduced the expression of XPG in human cells and studied what happened. These "knockdown" cells had a slew of problems associated with cell growth and cell cycle, as well as an increase in DNA double-strand breaks.
Next, the scientists set out to determine whether these problems are caused by a breakdown in the DNA replication process, in which two identical copies of DNA are made from one DNA molecule. They treated the cells with a chemical that causes replicative stress, and sure enough, the cells with reduced XPG levels were much more susceptible to the chemical.
This narrowed the search to homologous recombination repair, which is the most prevalent way of fixing broken replication forks. More research all but confirmed XPG's importance in homologous recombination repair: the scientists found that cells with reduced levels of XPG had a 50 percent loss in the repair process.
In a final step, the scientists mapped out mechanistically how XPG helps drive homologous recombination repair. Their results suggest that XPG swoops in during the initial stage of the process to clear chromatin of BRCA1, a protein that helps initiate repair. This step is thought to serve as a "reset button" for homologous recombination repair to allow the process to proceed. Almost nothing was known about this important step until now.
The scientists also found that XPG interacts with BRCA2 during a later stage in the process to load a protein called RAD51 onto DNA in need of repair. RAD51 is the "recombinase" protein that is essential for carrying out homologous recombination repair, but it needs help loading onto damaged DNA to perform its function.
"Until now, these direct interactions between XPG and BRCA1, BRCA2, and RAD51 during homologous recombination repair were unknown," says Cooper. "These interactions, and the greatly increased genome instability that occurs in XPG's absence, strongly suggest the protein is another important tumor-suppressor protein."
Source: DOE/Lawrence Berkeley National Laboratory
 Print Article
Print Article Mail to a Friend
Mail to a Friend
