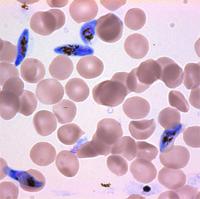
In blue are Plasmodium falciparum malaria parasites in the sexual, gametocyte stage of development. In red are uninfected red blood cells. Two teams have independently discovered that a single regulatory protein acts as the master genetic switch that triggers the development of male and female sexual forms (termed gametocytes) of the malaria parasite, solving a long-standing mystery in parasite biology with important implications for human health. The protein, AP2-G, is necessary for activating a set of genes that initiate the development of gametocytes -- the only forms that are infectious to mosquitos. The research also gives important clues for identifying the underlying mechanisms that control this developmental fate, determining whether or not a malaria parasite will be able to transmit the disease.
Even today, there is still a risk that malaria could be reintroduced into the United States and Europe, where malaria was largely eliminated by the 1950s. However, nearly half of the world's population -- 3.3 billion people -- currently live in 106 countries and territories that are at risk of malaria transmission, according to the U. S. Centers for Disease Control and Prevention. The World Health Organization estimates that there were 207 million cases of malaria and 627,000 deaths worldwide in 2012. Approximately 77 percent of those killed were children under the age of 5.
Malaria is caused by single-celled Plasmodium parasites. To survive and reproduce, these parasites have a rather complex lifecycle that involves three major stages. First, after a person is bitten by a parasite-carrying mosquito there is an initial infection in the liver, followed by the long-lasting red blood cell stage where the clinical symptoms of the malaria disease occur, and finally the mosquito stage, which is required to transmit the parasites to other people. All three stages are essential for the parasite to fully complete its developmental lifecycle. Surprisingly, the major blood stage form of the parasite that causes the terrible cycles of intense fevers associated with malaria in humans cannot be transmitted to mosquitos. For mosquito transmission to occur, the sexual form gametocytes, which are structurally distinct and have a very different program of gene and protein expression, have to form continually in the blood on a 2-day cycle. However, malaria parasites produce only a small number of sexual parasites per cycle – a double-edged strategy for the parasite that, on the plus side, ensures its survival during dry seasons when mosquitos are rare, but on the down side also represents a potentially vulnerable chokepoint. How parasites "decide" to produce sexual stages has been a mystery that has puzzled malaria researchers for years.
"Exciting opportunities now lie ahead for finding an effective way to break the chain of malaria transmission by preventing the malaria parasite from completing its full lifecycle," said Manuel Llinás, an associate professor of biochemistry and molecular biology at Penn State University. Llinás is the leader of an international team of scientists whose paper describing their research will be published in the journal Nature on the Advance Online Publication website, http://www.nature.com on 23 February 2014 along with a second paper, which describes related work led by Andy Waters (University of Glasgow) and Oliver Billker (Wellcome Trust Sanger Institute). Both manuscripts detail the role of the same AP2-G transcriptional regulator with remarkably similar findings -- despite the different groups' having worked with two highly diverged malaria parasites: Plasmodium falciparum, which causes the most severe form of human malaria and Plasmodium berghei, a commonly used model parasite infecting rodents.
"This sexual-stage bottleneck is an enticing target for interventions to prevent this comparatively small, yet critical number of sexual parasites from forming. If the sexual forms of the parasite never develop in an infected person's blood, then none will get into the mosquito's gut, and the mosquito will not be able to infect anyone else with malaria." Llinás said.
The study led by Llinás was initiated by experiments from Alfred Cortés' group at the Barcelona Centre for International Health Research, in which individual human malaria parasite clones from a single strain were found to have varying levels of the transcriptional regulator AP2-G, which mirrored varying levels of sexual stage (gametocyte) production. "The results were surprising because we found huge differences in the number of sexual-stage parasites produced by the different cell lines," Llinás said. One cell line produced ten times more than any of the others, most produced very few, and some produced none at all. Further experiments went on to show that the actual level of sexual-stage parasites produced by each parasite clone matched the proportion of individual cells specifically producing the AP2-G protein. "Our results perfectly correlate the expression of the ap2-g gene with the number of sexual-stage malaria parasites formed." Llinás said. 
An Anopheles mosquito takes a blood meal through the skin.
Because all these parasites share the identical DNA, or genetic makeup, yet the descendants of the individual cells produce significantly different numbers of sexual-stage parasites, Llinás said "we suspect that something outside the genes -- something other than a mutation in the DNA sequence -- is controlling the development of sexual-stage parasites." The research suggests that this phenomenon is not encoded directly in the parasite's DNA, but rather in other "epigenetic" differences between the parasites. In fact, Llinás said, "previous work has identified that a strong, repressing, epigenetic histone modification is present at the ap2-g gene and a few other locations in the malaria parasite genome." Cortés added: "For many years we have known that malaria parasites use epigenetic mechanisms to evade immune responses from the human host. Now we know that epigenetic mechanisms also regulate many other important processes in malaria parasite biology, including sexual differentiation".
When scientists discover a protein such as AP2-G that regulates a biological process, the typical next question is: and what other protein controls this regulator? "A beautiful aspect of our findings is that we don't need an upstream regulator for AP2-G. Instead, AP2-G can be activated by epigenetic mechanisms providing a plausible explanation for how low-level sexual conversion is triggered", says Cortés.
In what was originally a separate line of investigation, David Baker and Taane Clark and colleagues at the London School of Hygiene & Tropical Medicine and the Wellcome Trust Sanger Institute analyzed the whole-genome sequences of two P. falciparum laboratory strains that they knew were unable to produce gametocytes. Remarkably, they found that the only mutated, non-functional gene common to both strains was the ap2-g gene. David Baker said that "for more than 20 years my lab has been interested in identifying a malaria parasite gene underlying the switch to sexual development and finally the ap2-g locus, under epigenetic control, has come to light."
In the parallel study by the Waters and Billker groups, rodent malaria parasites that eventually lost their ability to produce sexual-stage parasites were selected over the course of a year in the laboratory (where the mosquito stage is not required). Using next-generation sequencing to identify the underlying mutations causing these effects, the groups found that the only common suspect was again the ap2-g gene -- the gene that codes for producing the AP2-G protein.
To confirm these observations, both studies disabled the ap2-g gene by cutting it out of the genome to remove its function from the parasite and the manipulated parasites indeed lost the ability to generate sexual-stage parasites. Furthermore, the parasites regained the ability to make gametocytes when the mutated gene in the selected rodent malaria parasites was repaired through gene therapy. Combined with other experiments, the results showed that sexual-stage malaria parasites are produced only when the AP2-G protein is in good working order. "Our research has demonstrated unequivocally that the AP2-G transcription-factor protein is essential for flipping the switch that initiates the transformation of malaria parasites in the blood from the asexual stage to the critical sexual stage of their life cycle," Llinás said.
The researchers agree these discoveries are exciting for the future of malaria research. Cells that make AP2-G enter sexual development, something that the groups are now able to control experimentally. "This opens the way to develop assays to screen for effective drugs that could disable commitment to sexual development and prevent transmission," comments Waters.
Billker adds "the discovery of AP2-G now gives us a new starting point to work out how the complex life cycle of malaria parasites is regulated by proteins within the parasite cells. It may even enable us to control parasite development in the laboratory."
The new ability to culture lots of sexual-stage malaria parasites will boost efforts to develop a sexual-stage vaccine that would help an infected person mount an immune response to prevent their malaria parasites from being transmitted to a mosquito -- effectively ending the life cycle for that person's batch of malaria parasites. "With the help of the next-generation technologies that we and other malaria researchers now are using, we are optimistic about more discoveries for malaria control that could occur soon -- even during the next 5 years," Llinás said.
Source : science@psu.edu
 Print Article
Print Article Mail to a Friend
Mail to a Friend
