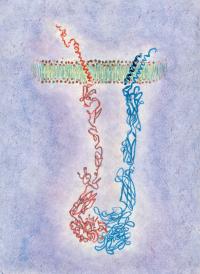
Artist rendering of integrin transmembrane protein. The red portion is the beta subunit; the blue is both the larger alpha subunit and the smaller bound peptide. Proteins, which form much of the molecular machinery required for life, are the targets of most drug molecules. One third of all proteins are membrane proteins – embedded within the cell’s fatty outer layer. While scientists can easily study the other two-thirds using such tools as antibodies, they have not had such methods to investigate the membrane-embedded portions of proteins.
To probe the secrets of these seemingly inaccessible proteins, researchers at the University of Pennsylvania School of Medicine have designed peptides that are able to bind to specific regions of transmembrane proteins, using computer algorithms, and information from existing protein sequence and structure databases. This study, which appears in the March 30 issue of Science, looks at how the binding of these designed peptides affects the crucial first steps in blood clotting.
"We can now actually interrogate parts of proteins within the membrane," says senior author William F. DeGrado, PhD, Professor of Biochemistry and Biophysics. "We used computer programs to design small proteins called peptides that can bind to only one of a number of closely related membrane proteins."
The researchers targeted two transmembrane proteins called integrins that influence the behavior of platelets, small blood cells important in clotting. One of these, the áIIbâ3 integrin, the most prominent integrin on platelets, is involved in making platelet aggregates, an important first step in the clotting process.
The other integrin, called áVâ3, behaves much like áIIbâ3, in that it causes platelets to stick to certain proteins on the outside of the cell. "We wanted to see if we could differentiate between the two integrins using two different peptides – and, in fact, we can," notes co-senior author Joel Bennett, MD, Professor of Medicine, who works with proteins and cells important in clotting.
When the designed peptide is inserted into the platelet membrane it binds to the portion of the integrin within the membrane, and subsequently perturbs another function in the clotting process downstream. "By having molecules that bind to the membrane-embedded portions of these proteins, we were able to address questions concerning the way that these proteins are regulated to cause clotting," explains co-first author Joanna Slusky, a doctoral student in the DeGrado laboratory.
"Therapeutics derived from this approach are a long way off, but this method allows us to now study these interactions that are so fundamental to the way in which cells cooperate to carry out essential functions," says Bennett. "In the future, this knowledge can provide insights for identifying novel drug targets." Source : University of Pennsylvania School of Medicine
 Print Article
Print Article Mail to a Friend
Mail to a Friend
