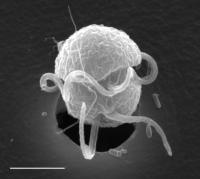
Scanning electron microscope image of Pfiesteria piscicida. Scale bar is 10 micrometers long. The hole in the background is a perforation in the filter that was used to collect the cells.
Credit: North Carolina State University Center for Applied Aquatic Ecology A team of researchers from the Hollings Marine Laboratory in Charleston, S.C., has uncovered a subtle chemical pathway by which a normally inoffensive algae, Pfiesteria piscicida, can suddenly start producing a lethal toxin. The discovery, reported last week in Environmental Science and Technology,* could resolve a long-standing mystery surrounding occasional mass fish kills on the East Coast.
Pfiesteria has been implicated for years in a series of otherwise unexplained episodes of mass fish death throughout its range from roughly Delaware to Alabama, particularly in the Neuse River in North Carolina and the Chesapeake Bay. The single-cell organism can experience explosive growth resulting in algae blooms in coastal waters. While it has been suspected not only in fish kills but in incidents of human memory loss and other environmental and health-related effects, no one has ever conclusively identified the actual mechanism. Attempts to grow lethal Pfiesteria in the laboratory have had inconsistent results.
The Hollings Marine Laboratory is a joint institution of the National Oceanic and Atmospheric Administration (NOAA), the National Institute of Standards and Technology (NIST), the South Carolina Department of Natural Resources, the College of Charleston, and the Medical University of South Carolina (MUSC). Lead researcher Peter Moeller of NOAA suspected that the presence or absence of heavy metals might be the missing factor accounting for Pfiesteria's lethality, and put together a multidisciplinary research team to identify the actual toxin and the conditions under which it is produced.
The work was complicated by the fact that the suspect toxin turns out to be highly unstable, decomposing rapidly once it's activated. Chemists from NIST and MUSC used an array of advanced spectroscopic techniques to determine that the toxin is characterized by the presence of copper-sulfur complexes. "NIST saved the day," Moeller said, "because we were working with only microgram and submicrogram quantities of the toxin. To not only determine that a metal was indeed present but even tell us which one really broke things open."
With that lead, Moeller's team established that the Pfiesteria cell can produce a copper-containing exotoxin--a toxin produced external to the cell itself--possibly as a mechanism to protect itself from copper in the environment. When exposed to sunlight or other environmental factors that can destabilize it, the toxin rapidly breaks down into short-lived free radicals, highly reactive chemical species believed to be the actual lethal agents. To observe the entire chain of events requires just the right combination of copper ions, temperature, light and Pfiesteria, explaining the difficulty researchers have had in reproducing the effect according to Moeller.
Although exotoxins like that produced by Pfiesteria are not common, the researchers observe, a number of other microalgae species are known to produce them, and under the right conditions in metal-rich waters they also might produce lethal variants.
Source : National Institute of Standards and Technology (NIST)
 Print Article
Print Article Mail to a Friend
Mail to a Friend
