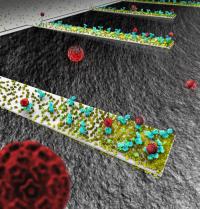
This rendition depicts an array of tiny, diving-boardlike devices called nanocantilevers. The devices are coated with antibodies to capture viruses, which are represented as red spheres.Researchers at Purdue University have made a discovery about the behavior of tiny structures called nanocantilevers that could be crucial in designing a new class of ultra-small sensors for detecting viruses, bacteria and other pathogens.
The nanocantilevers, which resemble tiny diving boards made of silicon, could be used in future detectors because they vibrate at different frequencies when contaminants stick to them, revealing the presence of dangerous substances. Because of the nanocantilever's minute size, it is more sensitive than larger devices, promising the development of advanced sensors that detect minute quantities of a contaminant to provide an early warning that a dangerous pathogen is present.
The researchers were surprised to learn that the cantilevers, coated with antibodies to detect certain viruses, attract different densities - or quantity of antibodies per area - depending on the size of the cantilever. The devices are immersed into a liquid containing the antibodies to allow the proteins to stick to the cantilever surface.
"But instead of simply attracting more antibodies because they are longer, the longer cantilevers also contained a greater density of antibodies, which was very unexpected," said Rashid Bashir, a researcher at the Birck Nanotechnology Center and a professor of electrical and computer engineering and biomedical engineering at Purdue University. The research also shows that the density is greater toward the free end of the cantilevers.
The engineers found that the cantilevers vibrate faster after the antibody attachment if the devices have about the same nanometer-range thickness as the protein layer. Moreover, the longer the protein-coated nanocantilever, the faster the vibration, which could only be explained if the density of antibodies were to increase with increasing lengths, Bashir said. The research group also proved this hypothesis using optical measurements and then worked with Ashraf Alam, a researcher at the Birck Nanotechnology Center and professor of electrical and computer engineering, to develop a mathematical model that describes the behavior.
The information will be essential to properly design future "nanomechanical" sensors that use cantilevers, Bashir said.
Findings are detailed in a research paper appearing online today (Monday, Aug. 28) in Proceedings of the National Academy of Sciences. The paper was authored by Amit K. Gupta, a former Purdue doctoral student working with Bashir and now a postdoctoral researcher at Harvard University; Pradeep R. Nair, a doctoral student in electrical and computer engineering; Demir Akin, research assistant professor of biomedical engineering; Michael Ladisch, Distinguished Professor of Agricultural and Biological Engineering with a joint appointment in the Weldon School of Biomedical Engineering; Steven Broyles, a professor of biochemistry; Alam and Bashir.
The work, funded by the National Institutes of Health, is aimed at developing advanced sensors capable of detecting minute quantities of viruses, bacteria and other contaminants in air and fluids by coating the cantilevers with proteins, including antibodies that attract the contaminants. Such sensors will have applications in areas including environmental-health monitoring in hospitals and homeland security. So-called "lab-on-a-chip" technologies could make it possible to replace bulky lab equipment with miniature sensors, saving time, energy and materials. Thousands of the cantilevers can be fabricated on a 1-square-centimeter chip, Bashir said.
The cantilevers studied in the recent work range in length from a few microns to tens of microns, or millionths of a meter, and are about 20 nanometers thick, which is also roughly the thickness of the antibody coating. A nanometer is a billionth of a meter, or approximately the length of 10 hydrogen atoms strung together.
A cantilever naturally "resonates," or vibrates at a specific frequency, depending on its mass and mechanical properties. The mass changes when contaminants land on the devices, causing them to vibrate at a different "resonant frequency, " which can be quickly detected. Because certain proteins attract only specific contaminants, the change in vibration frequency means a particular contaminant is present.
Ordinarily, when using cantilevers that are on a thickness scale of microns or larger, attaching mass causes the resonant frequency to decrease, which is the opposite of what occurs with the nanoscale-thickness cantilevers. Researchers believe the unexpected behavior is a result of the antibodies being about the same thickness as the ultra-thin nanocantilevers, meaning their vibration is more profoundly affected than a more massive cantilever would be by the attachment of the antibodies.
"The conclusion is that when the attached mass is as thick as the cantilever, then you not only affect the mass but you also affect a key property called the net stiffness constant and the resonant frequency can actually go up," Bashir said.
Gupta measured the cantilever's vibration frequency using an instrument called a laser Doppler vibrometer, which detects changes in the cantilever's velocity as it vibrates. The researchers then treated the antibodies with a fluorescent dye and took images of the proteins on the cantilever's surface, proving that the density increases with longer cantilevers.
Nair and Alam then developed a mathematical model to explain why the density increases as the area of the cantilever rises. The model uses a "diffusion reaction equation" to simulate the antibodies sticking to the cantilever's surface.
Source : Purdue University
 Print Article
Print Article Mail to a Friend
Mail to a Friend
