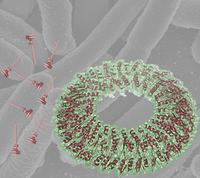
A composite figure showing a surface rendering of the critical EscJ inner-membrane ring structure representing the first crystallographic analysis of a major structural component of the Type III system. The structure is superposed on a background electron microscopic image of enterpathogenic E.coli, along with a low-resolution model of the intact needle as derived from earlier electron microscopic analysis. EscJ is localized to the terminal pair of stacked rings in the model. Image: Natalie StrynadkaThe bacteria that cause food poisoning, bubonic plague, and whooping cough all deploy the same weapon to infect the body. A molecular "syringe" sticks out of the bacteria, pokes a hole in a nearby cell, and squirts in venomous proteins that hijack the cell's machinery.
For the first time, Howard Hughes Medical Institute researchers have captured a detailed picture of the large doughnut-shaped base of the syringe barrel embedded in the bacterial membranes. The findings are reported in a paper in the June 2, 2005, issue of the journal Nature.
This first atomic picture of a major structural component of the hazardous molecular hypodermic may help scientists develop a new kind of drug that can disable the syringe and render disease-causing bacteria harmless while sparing beneficial bacteria. Currently, doctors must fight bacterial infections with antibiotics, which kill all bacteria, good and bad. Furthermore, the researchers are optimistic that drugs of this type might be effective against pathogens that are resistant to existing antibiotics.
"We believe this ring forms the foundation upon which all of the other components assemble," said senior author Natalie Strynadka, a HHMI international research scholar and associate professor of biochemistry at the University of British Columbia in Vancouver, Canada. "Without this assembly, there is no pathogenesis. This provides a real potential point of intervention."
The syringe is used almost exclusively by pathogenic bacteria, such as the Escherichia coli (E.coli) sometimes found in uncooked hamburgers, the Pseudomonas that cause life-threatening infections in the lungs of people with cystic fibrosis, and the plague bacillus, Yersinia pestis, that causes so-called black death. Many major plant pathogens also use the same piercing needle to puncture plant cells.
The barrel of the syringe spans the inner and outer membranes of Gram-negative bacteria, a major category of microbes that have multilayered cell walls. Two proteins at the tip of the needle drill into other cells. Each species of Gram-negative bacteria injects a distinctive blend of proteins that do different things to the cell and result in diverse diseases. The shared molecular machinery that injects the customized protein is called a type III secretion system.
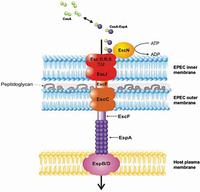
Twenty highly conserved proteins form an apparatus which spans the inner and outer membrane of the bacteria as well as that of the human host cell. Bacterial virulence proteins (purple spheres) are selectively secreted through the bacterial syringe into the host cell, where they manipulate host cell functions essential for subsequent pathogenicity. EscJ is the inner membrane ring which forms the platform upon which all other Type III secretion components assemble. Image: Natalie Strynadka"This is a major leap forward in understanding how type III systems work, because these things are so conserved among the rogues' gallery of Gram-negative bacteria," said co-author B. Brett Finlay, another HHMI international research scholar and the Peter Wall Distinguished Professor in the Michael Smith Laboratories at the University of British Columbia. "It's been a black box. This gives us an inside edge."
The new molecular model shows a circle of 24 identical interlocking molecules that hint at how the other syringe components may fit together. This large ring sits on top of the inner membrane of the Gram-negative bacterium, the portal where infectious proteins gather to exit the cell.
The new model is based on experiments by first author Calvin Yip, a graduate student in Strynadka's lab, using a technique called x-ray crystallography. The number of molecules was confirmed by mass spectroscopy analysis of intact type III secretion complexes in the lab of co-author Sam Miller at the University of Washington. Together, these experiments revealed details that confirm and enhance the blurry picture of the secretion system previously reported by other researchers relying on an electron microscope.
The protein ring, called EscJ, is a large, fatty molecule that had defied repeated attempts to grow it into the orderly crystals needed for detailed structural studies. One of the problems was that the ring's surface is glazed with positively charged atoms that push clones of themselves away like magnets repelling each other.
The breakthrough in the lab came when Yip made a mutant version to reduce some of the surface charge. That allowed the mutant EscJ molecules to line up in orderly arrays in a crystal. Researchers in Finlay's lab confirmed that the mutant protein could still form a functional syringe in bacteria. Based on the way the EscJ molecule is packed in the crystal, the scientists suspect that EscJ forms a ring that functions as a molecular platform for the assembly of the secretion system.
The greatest excitement about the new atomic structure comes from the potential to selectively target a type of disease-causing bacteria, the researchers said.
"Part of why this is so important is that antibiotics are becoming resistant and are not working anymore," Finlay said. "All the major pharmaceutical companies have pulled out. There are no new antibiotics in the drug development pipeline. We need different ways of thinking about going after bacteria."
Additionally. the crystal structure may illuminate more bacterial functions than their injection apparatus, Yip said. Several dozen genes clustered together on a region of the chromosome known as a "pathogenicity island" produce the components of the syringe, Yip explained. Interestingly, several of those genes closely resemble another set of genes that make up the flagella, the beating hairs that propel some bacteria.
Source : Howard Hughes Medical Institute
 Print Article
Print Article Mail to a Friend
Mail to a Friend
