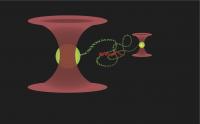
This graphic shows DNA strung between two beads, which are held in position by laser. It's been more than 50 years since James Watson and Francis Crick showed that DNA is a double helix of two strands that complement each other. But how does a short piece of DNA find its match, out of the millions of 'letters' in even a small genome? New work by researchers at the University of California, Davis, handling and observing single molecules of DNA, shows how it's done. The results are published online Feb. 8 by the journal Nature.
Defects in DNA repair and copying are strongly linked to cancer, birth defects and other problems.
"This is a real breakthrough," said Stephen Kowalczykowski, professor of microbiology and co-author on the paper with postdoctoral researcher Anthony Forget. "This is an issue that has been outstanding in the field for more than 30 years."
"It's the solution of one of the greatest needle-in-the-haystack problems in biology," said Professor Wolf-Dietrich Heyer, a UC Davis molecular biologist who also studies DNA repair but was not involved in this research.
"How can one double-stranded DNA break find its match in an entire genome, five billion base pairs in humans? Now we know the fundamental mechanism," Heyer said.
Forget and Kowalczykowski used technology developed in Kowalczykowski's lab over the past 20 years to trap lengths of DNA and watch, in real time, as the proteins involved in copying and repairing DNA do their work.
The first step in repairing a damaged piece of normally double-stranded DNA by a process called recombination is to strip it to a single strand. That single-stranded DNA then looks for a complementary sequence within an intact chromosome to use as a template to guide the repair.
How does a short, single-stranded piece of DNA find its exact matching partner out of perhaps millions of possibilities? In the 1970s, scientists discovered a protein, called RecA in bacteria and Rad51 in humans, which binds to the single-stranded DNA, forms an extensive filament and guides it to the right place in the chromosome.
"This is a very important aspect of chromosome maintenance," Kowalczykowski said. "Without it, your genome will start to scramble very quickly."
Defects in some proteins associated with DNA repair are associated with an increased risk of cancer – for example BRCA2, the breast cancer gene. But animals with defects in Rad51 don't even survive as embryos.
But how this search for DNA sequence compatibility works has been unclear. The RecA/DNA complex has to bump into and sample different stretches of DNA until it finds the right one, but the number of sequences to search is huge – it's like finding the proverbial needle in the haystack.
One model would be for RecA and its attached single-stranded DNA to slide along the intact duplex DNA until it gets to the right place. Or, if the DNA is in a coiled up form like a bowl of spaghetti, the RecA/DNA filament might be able to touch several different stretches of DNA simultaneously and thus shorten the time for the search.
Forget set out to test these ideas by stretching single molecules of duplex DNA between two tiny beads to make a dumbbell shape. Both beads were held in place by laser beams, but one of the beads could be steered around using the laser. Then he added the RecA assembled on single-stranded DNA to the DNA-dumbbells and watched to see how well they attached to the target DNA when it was stretched out, or relaxed and allowed to coil up.
"These are very complicated experiments to perform," Kowalczykowski said.
They found that the RecA complex attached most efficiently to the target DNA when it was in a relaxed, coiled form.
"The most efficient homology search is when the local DNA density is higher and the RecA-DNA filament can contact more areas of duplex DNA at the same time," Kowalczykowski said. "RecA doesn't slide along the DNA looking for a partner."
Consider a bowl of spaghetti, Kowalczykowski said. If you were looking for one tiny region on just one piece of spaghetti in the bowl, you could grab several strands at once and quickly examine each. But if the spaghetti were stretched out in one long piece, you could only touch one part of one piece at a time.
Kowalczykowski began working on the system for studying single molecules of DNA in 1991 with the late Ron Baskin, professor of molecular and cellular biology at UC Davis. In 2001, they demonstrated the technique by filming an enzyme called a helicase at work in real time unwinding the double helix of DNA. Since then, they have used the method to get new insights into the complex of proteins that copy and repair DNA.
Kowalczykowski's lab was also one of two UC Davis groups to purify the protein made by the BRCA2 gene, strongly associated with breast cancer. BRCA2, it turns out, loads Rad51 – the human equivalent of RecA in bacteria – onto DNA to search the human DNA for the correct region to use for repair. Source : University of California - Davis
 Print Article
Print Article Mail to a Friend
Mail to a Friend
