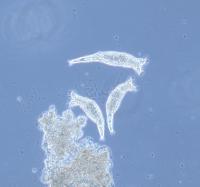
The bdelloid rotifer Philodina roseola. Bdelloids live in ephemeral aquatic habitats, such as temporary freshwater pools. Birds and bees may do it, but the microscopic animals called bdelloid rotifers seem to get along just fine without sex, thank you. What’s more, they have done so over millions of years of evolution, resulting in at least 370 species. These hardy creatures somehow escape the usual drawback of asexuality – extinction – and the MBL’s David Mark Welch, Matthew Meselson, and their colleagues are finding out how.
In two related papers published this week in Proceedings of the National Academy of Sciences (PNAS), the team proposes an interesting hypothesis: Bdelloid rotifers have been able to give up sex and survive because they have evolved an extraordinary efficient mechanism for repairing harmful mutations to their DNA.
“We think, in the bdelloid rotifer, genomic changes together with environmental changes have conspired to create something that is able to exist in the absence of sex,” says Mark Welch, an assistant scientist in the MBL’s Josephine Bay Paul Center.
Their results have medical implications, because DNA repair capacity is an important factor in cancer, inflammation, aging, and other human conditions.
In animals that do have sex, DNA repair is accomplished during meiosis, when chromosomes pair up (one from the father, one from the mother) and “fit” genes on one chromosome can serve as templates to repair damaged genes on the other chromosome. The bdelloid, though, always seems to reproduce asexually, by making a clone of itself. How then, does it cope with deleterious mutations?
In the first PNAS paper, MBL adjunct scientist Matthew Meselson and Eugene Gladyshev, both of Harvard University, demonstrate the enormous DNA repair capacity of bdelloid rotifers by zapping them with ionizing radiation (gamma rays), which has the effect of shattering its DNA into many pieces. “We kept exposing them to more and more radiation, and they didn’t die and they didn’t die and they didn’t die,” says Mark Welch. Even at five times the levels of radiation that all other animals are known to endure, the bdelloids were able to continue reproducing.
“Because there is no source of such intense ionizing radiation on Earth, except if we make it, there is no way these organisms could have evolved to be radiation resistant,” says Mark Welch. Instead, they propose that bdelloids’ DNA repair capacity evolved due to a different environmental adaptation – tolerance of extreme dryness.
Bdelloids, which live in ephemeral aquatic habitats such as temporary freshwater pools and on mosses, are able to survive complete desiccation (drying out) at any stage of their life cycle. They just curl up and go dormant for weeks, months, or years, and when water becomes available, they spring back to life. Mark Welch and his colleagues showed that desiccation, like ionizing radiation, breaks up the rotifers’ DNA into many pieces. Presumably, the same mechanisms they use to survive desiccation as part of their life cycle also protect them from ionizing radiation.
“That’s the next thing we are looking at. How are the bdelloids able to repair this many double-stranded breaks in their DNA? Do they have better enzymes, more enzymes?” Mark Welch says.
One feature that may confer exceptional DNA repair capacity on the bdelloids is described in the team’s second PNAS paper. Here, they give evidence that the bdelloid rotifer, like most animals, originally had two copies of each chromosome. But at some point in its evolution, it underwent a “whole-genome duplication,” giving it four copies of each chromosome and hence of each gene. Normally, lineages that undergo whole-genome duplication lose the duplicate genes over time. The bdelloid, though, has kept most of its duplicate genes throughout its evolutionary history.
“We believe they have kept most of their duplicate genes because they are serving as templates for DNA repair,” says Mark Welch. One possible result of DNA repair is gene conversion, in which the gene being repaired ends up having an identical DNA sequence to the gene repairing it. This can introduce the kinds of changes into the gene pool that sex usually does. (For example, a gene coding for brown eyes may repair a gene coding for blue eyes on its paired chromosome, and end up turning the blue-eye gene into a brown-eye one.)
“We think that gene conversion resulting from DNA repair resulting from adaptation to (desiccation in) its environment may provide enough of the advantages of sex that bdelloids can survive,” Mark Welch says.
Source : Marine Biological Laboratory
 Print Article
Print Article Mail to a Friend
Mail to a Friend
