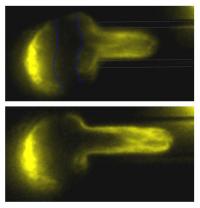
A stem cell nucleus flows like a soft plastic. The chromatin fibers are tagged yellow and a dark stripe is painted to help visualize the flow into a micro-capillary. Biophysicists at the University of Pennsylvania have discovered that the nuclei of human stem cells are particularly soft and flexible, rather than hard, making it easier for stem cells to migrate through the body and to adopt different shapes, but ultimately to put human genes in the correct nuclear ¡°sector¡± for proper access and expression.
Researchers pulled cell nuclei into microscopic glass tubes under controlled pressures and visualized the shear of the DNA and associated proteins by fluorescence microscopy. The study showed that nuclei in human embryonic stem cells were the most deformable, followed by hematopoietic stem cells, HSCs, that generate a wide range of blood and tissue cells. Both types of stem cells lack lamins A and C, two filamentous proteins that interact to stabilize the inner lining of the nucleus of most tissue cells. Lamins A and C stiffen cell nuclei and are expressed in cells only after gastrulation, when most stem cells generate the specific tissues of complex organisms.
The fluid-like character of the nucleus is shown to be set largely by the DNA and the DNA-attached proteins that form chromatin. The extent of deformation of the nucleus is further modulated by the lamina.
¡°Understanding the sensitivity of stem cells and their nuclei to external stresses has very practical implications in handling these cells as well as in technologies such as cloning in which nuclei are manipulated,¡± said Dennis Discher, a professor in Penn¡¯s School of Engineering and Applied Science and the Penn School of Medicine¡¯s Cell and Molecular Biology Graduate Group.
The study, published in the Oct. 2 issue of the Proceedings of the National Academy of Sciences, supports the theory that lamin proteins are responsible for much of the genomic ¡°lock-down¡± within differentiated cells. Differentiated cells, typified by muscle cells, fat cells and bone cells, all arise from stem cells that have committed to these specialized cell types by locking the DNA into a set pattern of gene expression.
To verify that lamin proteins were responsible for nuclear stiffness, the authors created a line of epithelial cells in which lamin filaments had been almost eliminated. Once as stiff as any other differentiated tissue cell derived from stem cells, the cell became as pliable as HSCs.
¡°Controlling structural proteins within the nucleus might lead to new means for controlling genomic regulatory factors and for generating stem cells from adult tissue cells,¡± J. David Pajerowski, lead author and a graduate student in Penn¡¯s School of Engineering and Applied Science, said.
Researchers also found that over time nuclear deformations in stem cells and hematopoietic cells became resistant to returning to their original shape, which provides evidence of plastic flow similar to that of wet clay in the hands of a sculptor. Continued application of force eventually pulled nuclei into irreversible forms in which genes were re-arranged and massaged into new nuclear locations. Researchers literally visualized the flow of chromatin, the structure that carries DNA, and found irreversible distortions occurring on a timescale of minutes, a long time compared to many other cell processes but short compared to the lifetimes of nuclei in our tissue cells.
Source : University of Pennsylvania
 Print Article
Print Article Mail to a Friend
Mail to a Friend
