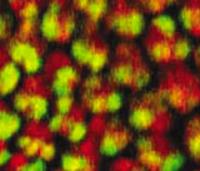
Photoreceptor cells (red) in the developing eye of a fruit fly initiate an unfolded protein response (yellow) to help them cope with a stressful environment. Misfolded proteins are implicated in many diseases, including neurodegenerative diseases, diabetes and types of blindness.
Credit: Rockefeller University When cells get stressed, their proteins go unfolded. It's a reaction with a straightforward name: the unfolded protein response. Now, new research from Rockefeller University shows that this phenomenon actually serves a protective role; rather than a sign that the cell has given up, it may be a mechanism by which the cells cope with adversity. The findings were reported as an advance online publication in the EMBO Journal on the Dec. 14.
Diabetes, cancer, and neurodegenerative diseases including Huntington's and Parkinson's are linked to unfolded proteins. But Hermann Steller, head of the Strang Laboratory of Apoptosis and Cancer Biology at Rockefeller and a Howard Hughes Medical Institute investigator, focused on autosomal dominant retinitis pigmentosa (ADRP), which causes blindness. Unfolded proteins linked to ADRP accumulate in the endoplasmic reticulum, an organelle where proteins are manufactured and packaged for transport to the cell surface, unlike some other forms of the unfolded protein response that occur in the cell's cytoplasm.
To understand what impact the unfolded protein response had on the cell, Hyung Don Ryoo, a former postdoc in the Steller lab, used a protein called xbp1, whose mRNA is alternatively spliced when the cell is stressed and the unfolded protein response is activated. Ryoo rigged xbp1 with a fluorescent marker that would light up whenever this alternatively spliced version of xbp1 was made, allowing him to detect every time the cell initiated an unfolded protein response.
"Our work shows that the unfolded protein response is a protective pathway, and therapeutically this is the type of pathway you want to boost to protect cells from stress induced death," says Steller, who is the Strang Professor at Rockefeller.
What's more, the researchers found that only cells that had endoplasmic reticulum stress, not cytoplasmic stress, made the fluorescently tagged xbp1 protein.
"Many researchers had bunched the stress response of the cytoplasmic and endoplasmic reticulum proteins together," says Steller. "Though we are not saying there couldn't be any cross-communication between these two pathways, I think our results show that the situation is considerably more complex than had been previously appreciated. The cell really knows where the stress is coming from, and whether the unfolded protein response is initiated in the endoplasmic reticulum or in the cytoplasm."
Using the tools that Ryoo developed, Steller hopes that more aspects of the unfolded protein response pathway can be illuminated. While they found that the unfolded protein response initially protects retinal cells from death in the fly model of ADRP, the cells eventually die, leading to blindness. How the cell death pathway becomes activated is still a mystery, and Steller makes the point that although blocking cell death is a worthwhile therapeutic strategy, blocking it globally is not. Scientists still need to understand the specific proteins involved to create the most effective therapy.
"When it comes to avoiding cancer or defense against virally infected cells, cell death is a good thing," says Steller. "But when cells are stressed, and as a result are dying too easily, blocking the pathways that lead to the activation of cell death would be an ideal way to fight disease. Using this new technique, we can now go step by step down the pathway, not only identifying proteins that are functionally relevant to cell death, but also finding which will make the most attractive targets for pharmacological development." Source : Rockefeller University
 Print Article
Print Article Mail to a Friend
Mail to a Friend
