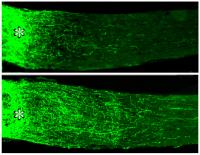
Rats whose retinas were treated with slow-release beads containing oncomodulin (plus a cofactor that boosts cells’ response to oncomodulin) showed dramatically increased growth of axons into the optic nerve (bottom)Researchers at Children's Hospital Boston have discovered a naturally occurring growth factor that stimulates regeneration of injured nerve fibers (axons) in the central nervous system. Under normal conditions, most axons in the mature central nervous system (which consists of the brain, spinal cord and eye) cannot regrow after injury. The previously unrecognized growth factor, called oncomodulin, is described in the May 14 online edition of Nature Neuroscience.
Neuroscientists Yuqin Yin, MD, PhD, and Larry Benowitz, PhD, who are also on the faculty of Harvard Medical School, did their studies in the optic nerve, which connects nerve cells in the eye's retina to the brain's visual centers, and is often used as a model in studying axon regeneration.
When oncomodulin was added to retinal nerve cells in a Petri dish, with known growth-promoting factors already present, axon growth nearly doubled. No other growth factor was as potent. In live rats with optic-nerve injury, oncomodulin released from tiny sustained-release capsules increased nerve regeneration 5- to 7-fold when given along with a drug that helps cells respond to oncomodulin. Yin, Benowitz and colleagues also showed that oncomodulin switches on a variety of genes associated with axon growth.
Benowitz, the study's senior investigator, believes oncomodulin could someday prove useful in reversing optic-nerve damage caused by glaucoma, tumors or traumatic injury. In addition, the lab has shown that oncomodulin works on at least one other type of nerve cell, and now plans to test whether it also works on the types of brain cells that would be relevant to treating conditions like stroke and spinal cord injury.
The current study builds on work Benowitz, Yin and colleagues published a few years ago. Studying the optic nerve, they found – quite by accident – that an injury to the eye activated axon growth: it caused an inflammatory reaction that stimulated immune cells known as macrophages to move into the eye.
"To make this finding clinically useful, we wanted to understand what was triggering the growth, so we could achieve nerve regeneration without causing an injury," Benowitz says.
Working in Benowitz's lab, Yin took a closer look and found that the macrophages secreted an essential but as-yet unidentified protein. Further studies revealed it to be oncomodulin, a little-known molecule first observed in association with cancer cells.
"Out of the blue, we found a molecule that causes more nerve regeneration than anything else ever studied," Benowitz says. "We expect this to spur further research into what else oncomodulin is doing in the nervous system and elsewhere."
For oncomodulin to work, it must be given along with an agent that raises cell levels of cyclic AMP, a "messenger" that initiates various cellular reactions. Increased cyclic AMP levels are needed to make the oncomodulin receptor available on the cell surface.
A two-pronged approach
Benowitz also notes that there is another side to the nerve-regeneration problem: overcoming agents that act as natural inhibitors of axon growth. These inhibitors are the subject of intense study by several labs, including that of Zhigang He, PhD, at Children's Hospital Boston.
In a study published in 2004, Benowitz and postdoctoral fellow Dietmar Fischer, PhD, collaborated with He to combine both approaches – overcoming inhibition and activating the growth state (by injuring the lens of the eye) – and achieved dramatic optic-nerve regeneration. Now that Benowitz has isolated oncomodulin, he believes even greater regeneration is possible by combining it with agents that counteract growth inhibitors.
"We're in the midst of an exciting era of research in nerve regeneration," Benowitz says. "There are a lot of promising leads in the area of blocking molecules that inhibit regeneration. But to get really strong regeneration, you also have to activate nerve cells' intrinsic growth state."
Source : Children's Hospital Boston
 Print Article
Print Article Mail to a Friend
Mail to a Friend
